Can Vitamin D Cause Gas in a Baby
Introduction
Vitamin D is a secosteroid and prohormone which can be obtained from food (every bit either vitamin Dii or Dthree), merely whose main source is endogenous production in the skin. This requires ultraviolet-B radiations (290-315 nm) of at least 18 mJ/cm2 intensity (one), inducing the formation of previtamin D3 from 7-dehydrocholesterol which later on converts to vitamin Diii (cholecalciferol) past body heat (2). Upon reaching the blood, vitamin D3 mainly binds to vitamin-D binding protein (DBP) and gets transported to the liver where it is transformed into its storage course calcidiol (25-hydroxyvitamin D3 or 25(OH)D3) through hydroxylation via the vitamin Diii 25-hydroxylases CYP2R1, CYP27A1 or CYP27B1, all members of the cytochrome P450 enzyme family (3, 4). 25(OH)D3 is the chief laboratory parameter to judge an individual's vitamin D status; concentrations <20 ng/ml (1ng/ml = ii,5 nmol/l) are considered every bit vitamin D deficiency, and 40-60 ng/ml equally ideal (2, 5). 25(OH)Dthree is metabolized in diverse tissues (predominantly the kidneys) to the biologically about active vitamin D hormone calcitriol (i,25-dihydroxyvitamin D or 1,25(OH)2D3) with another hydroxylation at the 1α position by CYP27A1 or CYP27B1 (3, 4). Too regulating calcium metabolism, ane,25(OH)2Diii has multiple pleiotropic furnishings, particularly within the allowed system, and is increasingly utilized not merely within prophylaxis, just also inside therapy of various diseases (ii, six, 7). In particular, binding of one,25(OH)2D3 to the vitamin D receptor (VDR) has been shown to inhibit the differentiation and proliferation of B and T helper (Th) lymphocytes, promoting the shift of an inflammatory to a more tolerant immune status which may explain the protective furnishings of vitamin D against autoimmune diseases [reviewed in (8)].
More recently, Slominski and colleagues have revealed alternative pathways of vitamin D metabolism mediated past the mitochondrial enzyme CYP11A1 which is able to hydroxylate the side chain of vitamin D2/Dthree (9–11). The main production of these reactions is 20-hydroxyvitamin D3 (20(OH)D3), which has a 20-30-fold lower concentration than 25(OH)D3 in human being serum (9, 11) and is the initial substrate for the formation of further hydroxy-derivatives such as 20,23(OH)twoDthree, 17,xx,23(OH)iiiD3 or 20,22(OH)iiDiii [reviewed in (12)]. These nonclassical vitamin D metabolites also act as hormones: besides being partial agonists of the VDR, they take loftier analogousness as agonists of the aryl hydrocarbon receptor (AhR) (13) and as inverse agonists of the retinoid-related orphan receptors (ROR) α and γ (14, fifteen). Notably, CYP11A1 is expressed in immune cells (16) and RORα and RORγ are expressed by inflammatory Th17 cells in which they synergistically regulate differentiation and inflammatory cytokine production (17). Th17-derived cytokines, notably interleukin (IL)17, have been implicated in the etiology of autoimmune disorders such every bit psoriasis (18) and multiple sclerosis (MS) (19). In addition, single nucleotide polymorphisms (SNPs) of the RORA cistron accept been associated with MS (twenty). The binding of ane,25(OH)iiD3, twenty(OH)D and other vitamin D hydroxy-metabolites to both RORα and RORγ, result in IL17 inhibition (14), thus providing another machinery distinct from VDR signaling how vitamin D may protect from, or convalesce symptoms of, autoimmune diseases.
For approximately 15 years, patients with autoimmune diseases, particularly MS, take been successfully treated using a loftier-dose vitamin D protocol. Considering this method has been developed by Prof. Dr. Cicero Coimbra in Sao Paolo, Brazil, it is ofttimes referred to as the "Coimbra protocol"; in Deutschland it is utilized since 2016. Underlying the Coimbra protocol is the hypothesis of a non-hereditary, just acquired grade of vitamin D resistance which this newspaper is going to examine.
A hallmark of acquired vitamin D resistance, if it exists, would be an elevated parathyroid hormone (PTH) concentration despite 25(OH)Diii levels being in the ideal range and thus indicating sufficient production of 1,25(OH)2D3. One cardinal role of i,25(OH)2D3 is to enhance intestinal calcium absorption. If ionized calcium concentrations in blood are low, the parathyroid glands release PTH which stimulates calcium release from bones. Furthermore, PTH increases the conversion of 25(OH)D3 into i,25(OH)iiDiii in the kidneys with subsequent release into circulation. PTH besides inhibits the tubular reabsorption of phosphate which in plough lowers the corporeality of water-insoluble calcium-phosphate salts and thus increases ionized calcium concentrations. In this way, PTH constitutes a direct feedback mechanism inside the vitamin D system. A physiological 25(OH)Diii level should thereby exist able to suppress PTH into the lower third of the reference range. In other words: If 25(OH)Diii levels are high, PTH should be low and vice versa. In patients with autoimmune diseases this negative feedback loop is disturbed. Based on these observations, Prof. Coimbra proposed the hypothesis of a vitamin D resistance.
The Hypothesis of Acquired Vitamin D Resistance
In two intervention studies, Carlberg and colleagues institute evidence that different individuals brandish a unlike molecular and biochemical response to the same dose of either long-term or single-bolus vitamin Dthree supplementation (21). Initially, in the VitDmet written report, 71 elderly prediabetic individuals were supplemented with either 0, 1600 or 3200 I.U. vitamin D3 daily over 5 months of Finnish winter (22). The focus of this work was on the furnishings of vitamin D3 supplementation on mRNA expression of twelve of the vitamin D-regulated genes and several vitamin D-affected laboratory parameters. With the aid of these biomarkers, the grouping was able to show in 2022 that even supposedly adequate high vitamin Dthree doses (3200 I.U.) were not able to exert the expected vitamin D-regulatory effects in all subjects. Focusing on the PTH feedback system lonely, a total of 25% of the patients showed no adequate response of this laboratory parameter. Regarding all 36 tested parameters, Carlberg et al. could cluster their patients in 24% depression responders, 51% mid responders and 25% loftier responders. In 2017, the group was able to reproduce these findings in the VitDbol study, in which a accomplice of healthy Finnish students received a eighty,000 I.U. bolus dose of vitamin D3 with similar low responder rates (23).
These data provided an in vivo confirmation that there exists a spectrum of different vitamin D responsiveness, with approximately 25% of a population not responding adequately to conventional vitamin D3 doses. They may require individually varying, only larger doses. Vitamin D resistance, as proposed by Prof. Coimbra and this commodity, could exist conceived as the extreme depression-response end of the vitamin D responsiveness spectrum. Accordingly, individuals existence vitamin D resistant would require very high doses of vitamin D3 supplementation to reach an adequate physiological response, such as a reduction of PTH concentrations or the down-regulation of an activated adaptive immune organization – the latter issue is important for the treatment of autoimmune diseases as discussed further below.
Indeed, the idea of vitamin D resistance has beginning been proposed in 1937 by Albright, Butler and Bloomberg based on the observation that in rare cases of rickets in children, very high doses of vitamin D were required to relieve symptoms (24). Information technology has later been shown that resistance to 1,25(OH)2D3 in such children is frequently caused past hereditary VDR defects, resulting in hypocalcemia, secondary hyperparathyroidism, rickets and in most one half of the patients baldness (25–27). In contrast to these cases of hereditary vitamin D resistance, which is very rare and already diagnosed in childhood, the hypothesis investigated in this paper concerns a non-hereditary, acquired form of vitamin D resistance which promotes the development of autoimmune diseases. Equally discussed in more particular below, such a course of vitamin D resistance could develop during crumbling based on an interaction between genetic susceptibility polymorphisms of the vitamin D arrangement and an aggregating of environmental factors additionally impairing the hormonal signaling of the vitamin D-derived hydroxy-metabolites. Acquired vitamin D resistance is therefore more common than hereditary vitamin D resistance according to the frequency of susceptibility polymorphisms and the rising incidence of autoimmune diseases.
Diagnosis of Vitamin D Resistance
In their article "The concept of the personal vitamin D response index", Carlberg and Haq (21) proposed the following: "A screening using a single vitamin D bolus treatment and measurements at days 0 and two paired with a simplified protocol of the molecular analysis used in the VitDbol study [… ] may exist the easiest style to identify persons with a low vitamin D response index" (21). Theoretically, people with acquired vitamin D resistance should also be found with this protocol as outliers with extremely low response indices. Yet, the majority of the biomarkers measured in the VitDbol written report tin only be determined in research laboratories. However, PTH provides a valuable and easy-to-measure out biomarker for the clinical therapist. As mentioned to a higher place i,25(OH)2D3 and PTH stand in a directly human relationship with each other. A high PTH concentration in turn is associated with a higher all-cause-mortality in epidemiological studies (28). Hence, the recommended optimal 25(OH)D3 level of >40ng/ml should correlate physiologically with an optimal PTH concentration. Because reference ranges and measurement units for PTH vary amidst different laboratories, it could be stated that a 25(OH)Dthree measurement >40 ng/ml should be associated with a PTH value in the middle of the lower third of its laboratory-specific reference range. In such a case, loftier PTH values are indicative for vitamin D resistance, assuming that dietary calcium and phosphate intake are acceptable and a differential diagnosis of hyperparathyroidism has been ruled out. For example, if a laboratory'southward reference range for PTH is 15-65 ng/ml, the centre of the lower third of that range would exist 23.3 ng/ml.
Furthermore, the constellation of high serum 1,25(OH)twoD3 concentrations with a concurrently physiological 25(OH)Diii concentration is known to occur in hereditary forms of vitamin D resistance or VDR knockout mice (29); it could therefore also exist indicative of acquired vitamin D resistance. The 1,25(OH)2D3 concentration is determined on the one hand by the action of the enzymes CYP2R1, CYP27A1 and CYP27B1 that catalyze production of one,25(OH)iiD3 and on the other mitt CYP24A1 which catalyzes one,25(OH)2D3 degradation. The inactivation of i,25(OH)2D3 occurs either systemically or locally within the cells of target tissues. Because PTH negatively regulates CYP24A1 and positively regulates the hydroxylases converting 25(OH)D3 to 1,25(OH)iiD3, the presence of vitamin D resistance, which results in low intestinal calcium absorption and thus PTH stimulation, would lead to a abiding acme of 1,25(OH)iiD3.
In case of a disturbed VDR response, e.grand., caused by SNPs, an increased 1,25(OH)2D3 concentration is urgently needed to maintain the integrity of VDR signaling. Therefore, an elevated PTH concentration tin result from vitamin D resistance mediated by dysfunctional VDR signaling. It follows that a decreased 25(OH)D3/1,25(OH)2D3 ratio could be perceived every bit a biomarker for vitamin D resistance. However, because PTH tiptop is causally responsible for an increased ane,25(OH)2D3 level, PTH is a clinically sufficient sensitive surrogate biomarker for vitamin D resistance (Figure 1). Especially since, according to our feel, a disturbed 25(OH)D3/1,25(OH)2Diii ratio is perceived as not sufficiently reliable. A reason could be that some cases of vitamin D resistance are based on SNPs in enzymes catalyzing product of 1,25(OH)2D3 such as CYP27B1, as originally proposed by Coimbra and his co-workers (xxx). In this case, PTH would exist elevated, but its activity on expression of this dysfunctional enzyme is not sufficient to heighten 1,25(OH)twoD3 levels.
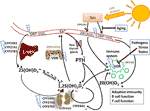
Effigy 1 Vitamin D metabolism and the sites of vitamin D resistance acquirement. Vitamin D3 is mainly produced in the skin through solar ultraviolet-B (UVB) radiation and gets transported through claret by binding to vitamin D bounden poly peptide (DBP). The liver is the systemic production site of 25(OH)Diii via the enzymes CYP2R1, CYP27A1 or CYP27B1. 25(OH)D3 then gets converted to the agile hormone i,25(OH)2D3 by a second hydroxylation which occurs mainly in the kidneys or in other tissues through paracrine production. 1,25(OH)2Diii binds to the vitamin D receptor (VDR) to promote intestinal calcium (Ca) assimilation and other Ca-mobilization pathways (e.g. in os), so that ionized Ca levels in serum ascension. The paraythroid glands sense Ca2+ levels, and secrete parathyroid hormone (PTH). PTH in turn promotes the production of 1,25(OH)2Diii, due east.g. by inhibiting CYP24A1 which catalyzes the showtime degradation step of 1,25(OH)2D3. Likewise 1,25(OH)iiD3, in that location are other vitamin D3-derived hydroxy-metabolites that exert hormone-like actions. For case, peel besides expresses CYP11A1, which promotes the conversion of vitamin Diii into 20(OH)D3, which has non-calcemic biological effects, for instance on immune cells. The blue flashes show where polymorphisms can interfere with normal vitamin D metabolism, building the basis for the development of caused vitamin D resistance. Boosted factors that impair vitamin D signaling are highlighted in bluish boxes.
Evolution of Vitamin D Resistance
Equally we have just illustrated, susceptibility for vitamin D resistance could arise from multiple polymorphisms of genes expressing for different proteins within the vitamin D system: the cytochrome-P450 enzymes (hydroxylases) needed for the conversion of vitamin D2/Diii into the activated hormone form (CYP2R1, CYP27A1 and CYP27B1), the DBP needed for vitamin D transport, the jail cell-surface receptor megalin-cubilin, which is the membrane receptor for the i,25(OH)2D3/DBP complex, the VDR itself or the recently discovered other receptors for vitamin D hydroxy derivatives such as RORα and RORγ. Indeed, inactivating polymorphisms of the CYP2R1 (31) or CYP27B1 (32) genes, promoter polymorphisms of CYP24A1 (33) or RORA (twenty), and SNPs of the VDR cistron (34–38) have all been associated with autoimmune diseases. All the same, equally suggested by research on hereditary vitamin D resistance, the VDR is probably the almost vulnerable office of the vitamin D metabolic system and constitutes the nigh likely or at least most potent manifestation of acquired vitamin D resistance.
The Vitamin D Receptor
The VDR is a steroid receptor expressed in almost all cell types of the human trunk, in item well-nigh allowed cells (38). The intracellular distribution of the VDR is ordinarily 75-80% in the nucleus, 15-xx% in the cytosol and three-5% in the plasma membrane (39). Bounden of 1,25(OH)2D3 induces translocation of cytosolic VDR into the nucleus in a dose-, fourth dimension- and temperature-dependent manner (40). In the nucleus, the liganded VDR forms a heterodimer with the unliganded retinoid-X receptor which so binds to DNA and recruits either coactivator or corepressor proteins and other transcription factors to exert gene regulatory functions (39). Due to this holding, the VDR is classified as a member of the nuclear receptor family. Other members of this "superfamily" of nuclear receptors are, among others, the thyroid receptor, the estrogen, progesterone and testosterone receptors, the retinoid-Ten receptor every bit well as the cortisol receptor (41). The high importance of the VDR is underscored past the slap-up number of genes that it regulates, both as a classical transcription cistron and through epigenetic furnishings (42). For instance, Seuter et al. take shown that one,25(OH)2Dthree binding to the VDR modulated the transcription of 1204 genes in the human monocytic cell line THP-1 over 24 hours, mostly by modulating the accessibility to their respective chromatin regions (43). The 46 chromatin strands, which mensurate up to 5 cm in length and have a diameter of approximately 0.01 mm, are thereby modified in a way that opens upward the target genes for reading (44). This epigenetic function of the VDR is exerted in almost all tissue types, which underlines its important role equally a regulatory protein well beyond its known function in calcium metabolism (42, 43).
Critically, several VDR SNPs have been revealed that are increasingly associated with multiple autoimmune diseases, either individually or equally haplotypes (34–38). For MS, e.g., a recent meta-analysis revealed a significant clan with rs731236 (TaqI) polymorphisms when comparing heterozygous (Tt) with homozygous (TT) genotypes (45). The rs731236 GG genotype was also a significant predictor of low vitamin D responsiveness in a supplementation written report of 100 Arab women, together with the rs7116978 (CYP2R1 factor) CC genotype (46). This study as well provided an overview showing that the rs7116978 G allele and rs7116978 C allele reached loftier population prevalence of upward to 40% or seventy%, respectively, depending on ethnicity (46). Information technology is known that certain haplotypes of VDR polymorphisms can influence its mRNA expression levels, stability and protein translation efficiency (47). Furthermore, work of Berth et al. who studied polymorphisms within the context of VDR binding to genes in human monocytes and dendritic cells, suggests that certain polymorphisms predispose to autoimmune diseases by perturbing VDR binding at autoimmune affliction take a chance gene variants (38). In other words, SNPs of the VDR may predispose to caused vitamin D resistance, which may become manifest during aging and lead to the development of an autoimmune disease, if additional factors accumulate that contribute to an impairment of VDR signaling. These factors will be reviewed in the next subsection.
The Vitamin D Receptor Is Regulated by Multiple Factors
With first evidence for a VDR in 550 1000000-year erstwhile boneless vertebrates, the VDR is a long-established regulatory poly peptide (48). From an evolutionary perspective it appears plausible that such a conserved and seminal protein is bailiwick to multiple influencing factors. We elaborate on this using the instance of the glucocorticoids in relation to the VDR.
Glucocorticoids (cortisol, cortisone) are excreted as part of a stress reaction (fight or flight, psychological stress) and ensure the supply of energy needed to solve the problem at hand. Within this context, the inhibition of the VDR appears logical, since it would ensure the shift of energy from the allowed to motor systems. Still, if the VDR is chronically inhibited, due east.g., upon long-term cortisone handling or chronic stress, this would explain the occurrence of pathological consequences such as osteoporosis. The clinically important immune-suppressive effect of glucocorticoids could be explained in part by the VDR blockade. Also the positive allowed modulatory issue of low-dose cortisol treatment could be due to an influence on the VDR.
The literature contains an increasing number of data confirming this hypothesis. For example, chronically elevated glucocorticoid levels have been shown to compromise the effects of 1,25(OH)iiDthree and promote the development of osteoporosis (49). Assistants of 10 mg prednisolone, an bogus glucocorticoid, for seven days to salubrious males resulted in enhanced renal calcium excretion, an elevation of biomarkers of bone degeneration and an increase in PTH levels. These effects could be compensated by the assistants of 1,25(OH)iiD3 (l). Another study showed that dexamethasone, another synthetic glucocorticoid, is able to potentiate the VDR-mediated transcription of CYP24A1 by a mechanism involving the recruitment of the glucocorticoid receptor to the CYP24A1 promoter region and its cooperation with C/EBPβ; since CYP24A1 catalizes the first step of i,25(OH)iiDiii deposition, dexamethasone effectively reduced i,25(OH)iiD3 concentrations (51). Finally, multiple putative glucocorticoid responsive elements could be identified inside the VDR gene, which suggest a regulation of VDR expression through endogenous glucocorticoids (52). This is consistent with type II diabetics having both college cortisol secretion (53) and lower VDR mRNA and protein levels (54) compared to healthy controls. In general, both inhibitory and activating tissue-specific furnishings on the VDR could be observed upon dexamethasone administration (52, 55).
Estrogens appear to positively stimulate vitamin D metabolism through a direct effect on the VDR (56, 57). An activation of the thyroid receptor, on the other hand, tin can have an inhibitory effect on vitamin D regulation via the VDR (58). These examples demonstrate that the response of the VDR tin can be modulated past a diverseness of physiological influences.
In addition, farther pathophysiological mechanisms take emerged during the evolution of life. Due to the profound influence of vitamin D on the immune system it appears reasonable from an evolutionary standpoint to view the VDR every bit a strategically of import target for pathological insults that aim to evade the immune arrangement. For multiple tumor cell lines an anti-proliferative issue of one,25(OH)2Dthree has been described (34). An attenuation of these anti-proliferative furnishings would confer a growth advantage for tumor cells. Along these lines, colorectal cancer cells have been shown to regulate the expression or responsiveness of the VDR (59). A blockade of the VDR was likewise revealed equally the pathophysiological mechanism exploited by osteosarcoma cells (sixty).
An increasing number of studies likewise depict the VDR as a strategic target of various pathogens. Lipopolysaccharides for example, which are sepsis inducing bacterial toxins, inhibit the expression of the VDR within THP-1 human monocytes (61).
In mammals, one of the central functions of the protein caspase-3 is the induction of programmed cell death (apoptosis). Thereby, information technology is maybe necessary to inhibit cellular vitamin D metabolism. Indeed, information technology was shown that caspase-3 is able to inhibit vitamin D metabolism by binding to and inactivating the VDR (62). Pathogens such as A. salmonicida, Legionella pneumophila, E. coli or Yersinia spp. in turn are able to interact with, or activate caspase-3 within cells, in this manner inhibiting the VDR and attenuating the immune response of their host (62, 63).
Blockade of the VDR has besides been described for other pathogens. A carefully conducted study has investigated the impact of Borrelia burgdorferi on the transcriptome of monocytes using whole genome microarrays [Supplementary Table S2 in (64)]. This technique revealed a sixty-fold downregulation of the VDR past live Borrelia. In man B lymphocytes, information technology was shown that their infection with Epstein-Barr-virus (EBV) inhibits VDR mRNA and protein expression (65). Yenamandra et al. confirmed this VDR blockade through EBV in B lymphocytes (66) and after showed that the EBNA-3 poly peptide is responsible for this blockade by its affinity to demark to the VDR (67). Finally, human being cytomegalovirus induced a 88% inhibition of VDR expression in infected cells, leading to a gradual increment of the CYP27B1 gene to 970%; notably, no downregulation of the VDR was observed for influenza or adenovirus infection (68). The mechanisms how pathogens disrupt vitamin D signaling may provide a missing link betwixt the clan of infections and autoimmune pathogenesis. Indeed, pathogen infections have been discussed as triggers of autoimmune diseases for a longer time (69). For example, infections with campylobacter, EBV or influenza viruses have been causally connected to the development of Guillain-Barré syndrome (70). Attention has also been paid to the link between EBV infection and MS development (71). As described above, blockade of the VDR by EBV could thereby provide a plausible underlying mechanism behind this link.
In summary, all these examples demonstrate a more or less potent influence on the vitamin D system via the VDR by physiological and pathophysiological influences. In particular, the pathogen-mediated temporary or chronic blockade of the VDR can be explained from an evolutionary view in which pathogens have developed ways to assault this primal target in order to downregulate their host'due south immune response. We thus propose the hypothesis that an interplay between acquired blockades of the VDR and polymorphisms affecting autoimmune disease susceptibility in either the VDR or other genes of the vitamin D organization are able to cause a progressively severe form of low vitamin D responsiveness, which nosotros refer to as acquired vitamin D resistance and which ultimately mediates the development of an autoimmune illness (Figure 2). Ii other autoimmune disease-related factors fit nicely into this picture, namely a lack of sun exposure and the aging process, as abdominal vitamin D3 absorption (72), endogenous pare production (73) and hydroxylation (74) all have been shown to decrease with aging. Finally, environmental toxins could besides exist integrated into this hypothesis if they interfere with vitamin D metabolism. For case aluminum, which has been found in loftier concentrations in brain tissue from MS patients (75), was able to decrease renal CYP27B1 activity in the chick (76). Hither, future inquiry on the effects of ecology toxins such as aluminum and cadmium on the vitamin D organization is urgently needed.
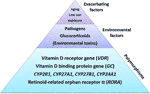
Figure 2 The etiology of acquired vitamin D resistance. Polymorphisms of genes inside the vitamin D organisation constitute the basis for a susceptibitly towards developing vitamin D resistance, and hence autoimmune diseases. Partial blockades of the vitamin D receptor through pathogens, glucocorticoids (chronic stress) and – putatively – environmental toxins such as heavy metals may collaborate with such a susceptibility then that vitamin D resistance emerges. Finally, depression sun exposure and aging, which correlate with autoimmune diseases likewise, volition exacerbate this state of affairs further.
Discussion
Consequences of Vitamin D Resistance
The expression of the VDR every bit a factor regulatory poly peptide in nearly all tissues of the man body suggests functional roles well beyond those classically associated with calcium metabolism. Along these lines, vitamin D is more and more than viewed equally essential for maintaining physiological homeostasis. Vitamin D deficiency is associated with a plethora of cardiovascular and metabolic diseases including cancer, hypertension, infectious and autoimmune diseases (ii, 7, 34). The proof of VDR expression in multiple prison cell types of the immune system such as CD4+/CD8+ lymphocytes, neutrophils and activated T-cell lymphocytes or antigen presenting cells (monocytes, macrophages or dendritic cells) underlines the importance of this receptor within this system (38). Mechanistically, multiple unlike furnishings of i,25(OH)twoDiii on the immune system accept been described, including, just not express to, chemotaxis, phagocytosis, proliferation, differentiation, cytokine production, antigen presentation, antibiotic, cathelicidin and hydrogen peroxide production and memory cell buildup (34). Only recently, Carlberg et al. identified a number of key genes of the allowed system which are expressed by the VDR upon 1,25(OH)2D3 activation (48). Likewise genes whose proteins play an essential role within the innate immune organization by producing antimicrobial peptides, another group of proteins was identified which are closely connected to autoimmune processes. An example is the transmembrane poly peptide Ninjurin1 (NINJ1) which is regulated by one,25(OH)2Dthree. NINJ1 is involved in transmigration of antigen-presenting cells across the blood-brain barrier and in the pathogenesis of MS (77).
In that location also is an epigenetic influence of the VDR on genes of the immune system. An in vivo proof-of-principle study investigated chromatin accessibility in monocytes obtained from a human subject field before and after a vitamin D bolus dose of 2000 µg (80,000 I.U.) (78). The bolus dose, which raised 25(OH)D3 levels by 7.6 ng/ml later on ii days, resulted in a significant opening or endmost of hundreds of gene loci, the most prominent of which belonged to the man leucocyte antigen (HLA) organisation coded on chromosome six. With the help of antigen-presenting proteins of the HLA system, the immune system is able to differentiate betwixt endogenous and exogenous proteins. Therefore, a dysfunction within the HLA arrangement predisposes for the development of autoimmune diseases. Polymorphisms of the HLA system for example found the most meaning genetic influence on MS (79). Interestingly, a vitamin D responsive element could be identified at a factor within the HLA-DRB1 region, which is closely associated with the evolution of MS (80). Likewise the epigenetic influence, this finding also underlines the transcriptional effect of vitamin D on the pathogenesis of this disease.
Looking across the box of autoimmune diseases to the mounting show of Vitamin D as a player in tumor development (81, 82), meaning associations between SNPs of vitamin D system genes and mutual types of cancer (prostate, breast, colorectal and skin cancer) take been reported (83, 84), suggesting a similar predisposing role of SNPs for cancer as for autoimmune diseases. In these cases, a partial blockade of the VDR by pathogens could be some other pathway within the established infectious etiology of cancer (85, 86).
In summary, there is much plausibility for caused vitamin D resistance playing a pathological role in the development of autoimmune diseases, in this manner providing an important component for our understanding and explanation of these diseases.
Therapy of Vitamin D Resistance
Currently no causal and therefore reliable therapies exist for correcting a blockade of the VDR. The just constructive therapy to engagement is the high-dose vitamin D protocol, known subsequently its inventor as the Coimbra protocol (30). With upwards to approximately k I.U. vitamin Dthree per kg body weight, the highest therapeutic vitamin D starting doses are thereby administered for the treatment of MS. For other autoimmune diseases much lower doses appear sufficient (Table ane). Hypercalcemia may be a major concern raised against this protocol. However, as discussed in more detail below, the vitamin D resistance appears to confer an intrinsic protection confronting hypercalcemia. In addition, the therapy requires the patient to take some precautionary measures. Too the avoidance of milk products and a minimum fluid intake of 2.5 50/solar day, patients will need to consistently monitor multiple blood parameters and undergo regular sonographic checks of their kidneys. Practically, this requires close and regular contact with a certified Coimbra practitioner.

Tabular array 1 Initial vitamin Diii doses used within the Coimbra protocol for the treatment of autoimmune diseases.
The Coimbra protocol follows a long medical tradition in which receptor resistances are compensated by applying higher doses. This is exemplified using a simplified analogy of insulin resistance therapy:
The current practice in type 2 diabetes direction consists of calculating the required external insulin dose according to blood glucose levels. Thereby it is nearly irrelevant how high the injected insulin dose actually is. It is the correction of the target parameter, i.due east. claret glucose, which is pivotal. To achieve this goal, total daily insulin doses exceeding 100 I.U. can exist utilized (87). These are doses that would normally be toxic in the absence of an underlying insulin resistance. The diabetic patient is to some extent protected against astringent side effects of what would commonly exist considered an overdose, because this dose simply acts physiologically – provided the practical doses are calculated correctly and there is close supervision by a doctor.
Although we acknowledge that the causes of insulin resistance are very different from those of vitamin D resistance, and loftier-dose insulin injections crusade insufficiently more side effects than high-dose vitamin D3 injections, the above illustration illustrates the principles of the Coimbra protocol: The calculation of the required vitamin D dose depends on the grade of vitamin D resistance and the stage of the particular disease and incorporates the PTH concentration in illustration to the insulin/claret glucose example. Provided shut supervision by an experienced physician, the applied high doses of vitamin D3 are causing neither hypercalcemia nor kidney damage. They will only exert physiological effects – in this fashion, the underlying vitamin D resistance acts protective against what would normally be considered a potentially toxic dose. In Supplementary Table 1 nosotros provide data collected in the corresponding author's practise (DL) revealing no case of calcium circuit in a cohort of 41 patients observed over at least 6 months. The development of their PTH concentrations is plotted in Figure iii and shows a significant decline at three months follow-up.
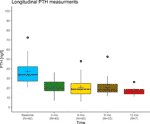
Figure 3 Longitudinal PTH measurements in a cohort of 41 relapsing-remitting multiple sclerosis patients treated by the corresponding author (DL) with the Coimbra protocol. Black lines indicate the median, blue crosses the hateful. At three months follow-up, PTH levels had dropped significantly compared to the baseline measurement (Wilcoxon rank sum test, p<0.001), while there were no significant differences between any of the follow-up measurements. The reduction of PTH concentrations into the middle of the lower tertiary of its laboratory-specific reference range is judged every bit an indicator for overcoming vitamin D resistance.
However, one case of a 39-year quondam symptomatic MS patient with hypercalcemia and renal insufficiency has been reported by a Swiss team of physicians (88). After 7 months on the Coimbra protocol with 100,000 I.U. of vitamin D3 daily, his calcium concentration at infirmary admission was three.0 mmol/L, his 25(OH)D3 level 697 ng/ml (1742 nmol/50), and his PTH was 96 ng/50. The PTH level was thus much higher than typically observed on the Coimbra protocol (Figure 3). A possible caption is that the patient was a carrier of the Multiple Endocrine Neoplasia, type 1 (MEN1) gene and had a history of elevated PTH levels already prior to starting the Coimbra protocol. Although this case highlights the possibility of high-dose vitamin D toxicity within the Coimbra protocol, it appears to be very rare. Even so, it too highlights the need for a diagnostic assessment of hyperparathyroidism prior to initiating the protocol equally well as a regular endocrinological monitoring during therapy. It is noteworthy that the patient described in the instance report wanted the authors to provide a brief account of his own standpoint according to which he and other patients he knew had improved their MS symptoms significantly on the protocol (88).
Such subjective improvements of autoimmune affliction patients likewise as condom data have also been collected within the network of Coimbra protocol physicians in Germany; these will be subject to future publications.
Finally, nosotros would briefly like to mention the opinion held past some therapists that vitamin D administration is counterindicated in patients with vitamin D resistance. This hypothesis is inconsistent with the medical experience and understanding according to which resistances tin be compensated with normal-to-high doses as described above. In particular, it is inconsistent with the experience gained past many Coimbra protocol physicians specialized in high dose vitamin D therapy that accept nerveless many treatment successes thus far.
In summary, we have reviewed bear witness for the hypothesis of an acquired form of vitamin D resistance, developing on the basis of a genetic susceptibility from sure SNPs within the vitamin D system and its interplay with chronic stress and/or pathogen infections that are able to partially cake the VDR. Other factors that have been associated with autoimmune diseases such as depression sun exposure, aging or environmental toxins could easily be integrated into this hypothesis since they would further exacerbate developing vitamin D resistance arising from the described mechanisms (Figure 2).
The hypothesis of acquired vitamin D resistance thus provides a plausible pathomechanism for the development of autoimmune diseases. We consider its therapeutic exploitation by loftier-dose vitamin D assistants as a promising arroyo. Our key messages reflecting the knowledge about vitamin D resistance and its treatment are summarized in Table two.

Table 2 Cardinal points discussed in this paper.
Information Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material, farther inquiries can be directed to the corresponding authors.
Author Contributions
DL and RJK drafted the initial manuscript. DL and BS collected the information. FS and JS edited the manuscript. All authors contributed to the commodity and approved the submitted version.
Disharmonize of Involvement
DL and BS are certified Coimbra practitioners. BS owns the practise that apply the here described Coimbra protocol to patients with autoimmune diseases.
The remaining authors declare that the research was conducted in the absense of any commercial or financial relationships that could be construed as a potential conflict of interest.
Supplementary Material
The Supplementary Material for this article can exist found online at: https://www.frontiersin.org/manufactures/10.3389/fimmu.2021.655739/full#supplementary-material
References
1. Matsuoka LY, Wortsman J, Haddad JG, Hollis BW. In vivo threshold for cutaneous synthesis of vitamin D3. J Lab Clin Med (1989) 114:301–5.
Google Scholar
ii. Gröber U, Spitz J, Reichrath J, Kisters Thou, Holick MF. Vitamin D: Update 2013. From rickets prophylaxis to general preventive healthcare. Dermatoendocrinol (2013) 5:331–47. doi: 10.4161/derm.26738
CrossRef Full Text | Google Scholar
3. Sawada Due north, Sakaki T, Ohta K, Inouye K. Metabolism of vitamin D3 by human CYP27A1. Biochem Biophys Res Commun (2000) 273:977–84. doi: 10.1006/bbrc.2000.3050
CrossRef Full Text | Google Scholar
iv. Jones One thousand, Prosser DE, Kaufmann M. Cytochrome P450-mediated metabolism of vitamin D. J Lipid Res (2014) 55:13–31. doi: ten.1194/jlr.R031534
CrossRef Full Text | Google Scholar
five. Holick MF. Vitamin D Status: Measurement, Interpretation, and Clinical Application. Ann Epidemiol (2009) 19:73–8. doi: ten.1016/j.annepidem.2007.12.001
CrossRef Full Text | Google Scholar
six. Gröber U, Kisters Thousand, Spitz J, Adamietz IA. Vitamin D in der onkologischen Intervention: Update 2015. Dtsch Z Für Onkol (2015) 47:173–7. doi: 10.1055/s-0035-1547572
CrossRef Full Text | Google Scholar
7. Grant WB, Lahore H, McDonnell SL, Baggerly CA, French CB, Aliano JL, et al. Show that vitamin d supplementation could reduce risk of influenza and covid-xix infections and deaths. Nutrients (2020) 12:988. doi: 10.3390/nu12040988
CrossRef Full Text | Google Scholar
8. Murdaca G, Tonacci A, Negrini S, Greco Thousand, Borro M, Puppo F, et al. Emerging function of vitamin D in autoimmune diseases: An update on evidence and therapeutic implications. Autoimmun Rev (2019) 18:102350. doi: x.1016/j.autrev.2019.102350
CrossRef Full Text | Google Scholar
9. Slominski AT, Kim T, Shehabi HZ, Semak I, Tang EKY, Nguyen MN, et al. In vivo evidence for a novel pathway of vitamin D3 metabolism initiated by P450scc and modified by CYP27B1. FASEB J (2012) 26:3901–15. doi: 10.1096/fj.12-208975
CrossRef Full Text | Google Scholar
10. Slominski AT, Kim TK, Shehabi HZ, Tang EKY, Benson HAE, Semak I, et al. In vivo production of novel vitamin D2 hydroxy-derivatives by human being placentas, epidermal keratinocytes, Caco-2 colon cells and the adrenal gland. Mol Cell Endocrinol (2014) 383:181–92. doi: 10.1016/j.mce.2013.12.012
CrossRef Full Text | Google Scholar
xi. Slominski AT, Kim TK, Li Due west, Postlethwaite A, Tieu EW, Tang EKY, et al. Detection of novel CYP11A1-derived secosteroids in the human epidermis and serum and pig adrenal gland. Sci Rep (2015) 5:14875. doi: 10.1038/srep14875
CrossRef Full Text | Google Scholar
12. Slominski AT, Li W, Kim TK, Semak I, Wang J, Zjawiony JK, et al. Novel activities of CYP11A1 and their potential physiological significance. J Steroid Biochem Mol Biol (2015) 151:25–37. doi: 10.1016/j.jsbmb.2014.11.010
CrossRef Total Text | Google Scholar
thirteen. Slominski AT, Kim TK, Janjetovic Z, Brożyna AA, Żmijewski MA, Xu H, et al. Differential and overlapping effects of 20,23(OH)2 D3 and ane,25(OH)2 D3 on gene expression in man epidermal keratinocytes: Identification of AHR equally an alternative receptor for twenty,23(OH)2 D3. Int J Mol Sci (2018) 19:3072. doi: 10.3390/ijms19103072
CrossRef Full Text | Google Scholar
14. Slominski AT, Kim TK, Takeda Y, Janjetovic Z, Brozyna AA, Skobowiat C, et al. RORα and ROR γ are expressed in man skin and serve as receptors for endogenously produced noncalcemic 20-hydroxy- and 20,23-dihydroxyvitamin D. FASEB J (2014) 28:2775–89. doi: 10.1096/fj.thirteen-242040
CrossRef Total Text | Google Scholar
15. Slominski AT, Kim TK, Hobrath JV, Oak ASW, Tang EKY, Tieu EW, et al. Endogenously produced nonclassical vitamin D hydroxy-metabolites human action as "biased" agonists on VDR and inverse agonists on RORα and RORγ. J Steroid Biochem Mol Biol (2017) 173:42–56. doi: x.1016/j.jsbmb.2016.09.024
CrossRef Full Text | Google Scholar
16. Slominski RM, Tuckey RC, Manna PR, Jetten AM, Postlethwaite A, Raman C, et al. Extra-adrenal glucocorticoid biosynthesis: implications for autoimmune and inflammatory disorders. Genes Immun (2020) 21:150–68. doi: 10.1038/s41435-020-0096-6
CrossRef Full Text | Google Scholar
17. Yang XO, Pappu BP, Nurieva R, Akimzhanov A, Kang HS, Chung Y, et al. T Helper 17 Lineage Differentiation Is Programmed by Orphan Nuclear Receptors RORα and RORγ. Immunity (2008) 28:29–39. doi: 10.1016/j.immuni.2007.eleven.016
CrossRef Full Text | Google Scholar
eighteen. Peiser M. Role of Th17 cells in skin inflammation of allergic contact dermatits. Clin Dev Immunol (2013) 2013:261037. doi: 10.1155/2013/261037
CrossRef Full Text | Google Scholar
nineteen. Tzartos JS, Friese MA, Craner MJ, Palace J, Newcombe J, Esiri MM, et al. Interleukin-17 production in central nervous system-infiltrating T cells and glial cells is associated with active disease in multiple sclerosis. Am J Pathol (2008) 172:146–55. doi: 10.2353/ajpath.2008.070690
CrossRef Full Text | Google Scholar
xx. Eftekharian MM, Noroozi R, Sayad A, Sarrafzadeh S, Toghi M, Azimi T, et al. RAR-related orphan receptor A (RORA): A new susceptibility gene for multiple sclerosis. J Neurol Sci (2016) 369:259–62. doi: x.1016/j.jns.2016.08.045
CrossRef Full Text | Google Scholar
21. Carlberg C, Haq A. The concept of the personal vitamin D response alphabetize. J Steroid Biochem Mol Biol (2018) 175:12–vii. doi: 10.1016/j.jsbmb.2016.12.011
CrossRef Total Text | Google Scholar
22. Saksa N, Neme A, Ryynänen J, Uusitupa Thou, De Mello VDF, Voutilainen S, et al. Dissecting high from low responders in a vitamin D3 intervention written report. J Steroid Biochem Mol Biol (2015) 148:275–82. doi: x.1016/j.jsbmb.2014.xi.012
CrossRef Full Text | Google Scholar
23. Seuter S, Virtanen JK, Nurmi T, Pihlajamäki J, Mursu J, Voutilainen Southward, et al. Molecular evaluation of vitamin D responsiveness of healthy young adults. J Steroid Biochem Mol Biol (2017) 174:314–21. doi: ten.1016/j.jsbmb.2016.06.003
CrossRef Total Text | Google Scholar
24. Albright F, Butler AM, Bloomberg Due east. RICKETS RESISTANT TO VITAMIN D THERAPY. Am J Dis Child (1937) 54:529–47. doi: 10.1001/archpedi.1937.01980030073005
CrossRef Full Text | Google Scholar
25. Liberman UA, Eil C, Marx SJ. Resistance to i,25-dihydroxyvitamin D. Association with heterogeneous defects in cultured skin fibroblasts. J Clin Invest (1983) 71:192–200. doi: 10.1172/JCI110759
CrossRef Full Text | Google Scholar
26. Malloy PJ, Eccleshall TR, Gross C, Van Maldergem L, Bouillon R, Feldman D. Hereditary vitamin D resistant rickets caused past a novel mutation in the vitamin D receptor that results in decreased affinity for hormone and cellular hyporesponsiveness. J Clin Invest (1997) 99:297–304. doi: 10.1172/JCI119158
CrossRef Full Text | Google Scholar
28. Schierbeck LL, Jensen TS, Blindside U, Jensen One thousand, Køber L, Jensen JEB. Parathyroid hormone and vitamin D—markers for cardiovascular and all cause mortality in heart failure. Eur J Eye Neglect (2011) 13:626–32. doi: ten.1093/eurjhf/hfr016
CrossRef Full Text | Google Scholar
29. Bouillon R, Verstuyf A, Mathieu C, Van Cromphaut S, Masuyama R, Dehaes P, et al. Vitamin D resistance. Best Pract Res Clin Endocrinol Metab (2006) 20:627–45. doi: x.1016/j.beem.2006.09.008
CrossRef Full Text | Google Scholar
thirty. Finamor DC, Sinigaglia-Coimbra R, Neves LCM, Gutierrez M, Silva JJ, Torres LD, et al. A pilot written report assessing the effect of prolonged assistants of high daily doses of vitamin D on the clinical course of vitiligo and psoriasis. Dermatoendocrinol (2013) 5:222–34. doi: 10.4161/derm.24808
CrossRef Full Text | Google Scholar
31. Ramos-Lopez E, Brück P, Jansen T, Herwig J, Badenhoop M. CYP2R1 (vitamin D 25-hydroxylase) gene is associated with susceptibility to type 1 diabetes and vitamin D levels in Germans. Diabetes Metab Res Rev (2007) 23:631–6. doi: 10.1002/dmrr.719
CrossRef Total Text | Google Scholar
32. Sundqvist Eastward, Bäärnhielm 1000, Alfredsson 50, Hillert J, Olsson T, Kockum I. Confirmation of association between multiple sclerosis and CYP27B1. Eur J Hum Genet (2010) 18:1349–52. doi: ten.1038/ejhg.2010.113
CrossRef Full Text | Google Scholar
33. Hallau J, Hamann L, Schumann RR, Worm Chiliad, Heine Chiliad. A promoter polymorphism of the vitamin D metabolism gene Cyp24a1 is associated with astringent atopic dermatitis in adults. Acta Derm Venereol (2016) 96:169–72. doi: 10.2340/00015555-2226
CrossRef Full Text | Google Scholar
34. Bizzaro G, Antico A, Fortunato A, Bizzaro Due north. Vitamin D and autoimmune diseases: Is vitamin D receptor (VDR) polymorphism the culprit? Isr Med Assoc J (2017) 19:438–43.
Google Scholar
35. Mory DB, Gabbay MAL, Rocco ER, Kasamatsu T, Crispim F, Miranda WL, et al. Loftier frequency of Vitamin D receptor gene polymorphism FokI in Brazilian Type 1 diabetes mellitus patients with clinical autoimmune thyroid illness. Diabetol Metab Syndr (2016) 8:29. doi: 10.1186/s13098-016-0145-five
CrossRef Full Text | Google Scholar
36. Gao XR, Yu YG. Meta-Analysis of the association between vitamin D receptor polymorphisms and the take chances of autoimmune thyroid illness. Int J Endocrinol (2018) 2018:2846943. doi: 10.1155/2018/2846943
CrossRef Total Text | Google Scholar
37. Marini F, Falcini F, Stagi Due south, Fabbri Southward, Ciuffi Due south, Rigante D, et al. Written report of vitamin D status and vitamin D receptor polymorphisms in a accomplice of Italian patients with juvenile idiopathic arthritis. Sci Rep (2020) 10:17550. doi: ten.1038/s41598-020-74861-9
CrossRef Total Text | Google Scholar
38. Berth DR, Ding N, Parnell GP, Shahijanian F, Coulter Southward, Schibeci SD, et al. Cistromic and genetic evidence that the Vitamin D receptor mediates susceptibility to latitude-dependent autoimmune diseases. Genes Immun (2016) 17:213–9. doi: 10.1038/gene.2016.12
CrossRef Full Text | Google Scholar
39. Norman AW, Henry HL. Hormones. 3rd ed. London: Academic Press (2015).
Google Scholar
xl. Racz A, Barsony J. Hormone-dependent translocation of vitamin D receptors is linked to transactivation. J Biol Chem (1999) 274:19352–60. doi: x.1074/jbc.274.27.19352
CrossRef Full Text | Google Scholar
41. Carlberg C, Campbell MJ. Vitamin D receptor signaling mechanisms: Integrated deportment of a well-defined transcription cistron. Steroids (2013) 78:127–36. doi: x.1016/j.steroids.2012.x.019
CrossRef Full Text | Google Scholar
43. Seuter S, Neme A, Carlberg C. Epigenome-broad effects of Vitamin D and their impact on the transcriptome of human monocytes involve CTCF. Nucleic Acids Res (2016) 44:4090–104. doi: x.1093/nar/gkv1519
CrossRef Full Text | Google Scholar
44. Nurminen V, Neme A, Seuter S, Carlberg C. The touch on of the vitamin D-modulated epigenome on VDR target gene regulation. Biochim Biophys Acta - Gene Regul Mech (2018) 1861:697–705. doi: 10.1016/j.bbagrm.2018.05.006
CrossRef Full Text | Google Scholar
45. Imani D, Razi B, Motallebnezhad Chiliad, Rezaei R. Association betwixt vitamin D receptor (VDR) polymorphisms and the adventure of multiple sclerosis (MS): An updated meta-analysis. BMC Neurol (2019) 19:339. doi: ten.1186/s12883-019-1577-y
CrossRef Full Text | Google Scholar
46. Tomei S, Singh P, Mathew R, Mattei V, Garand M, Alwakeel Chiliad, et al. The office of polymorphisms in vitamin D-related genes in response to vitamin D supplementation. Nutrients (2020) 12:2608. doi: x.3390/nu12092608
CrossRef Full Text | Google Scholar
47. Valdivielso JM, Fernandez E. Vitamin D receptor polymorphisms and diseases. Clin Chim Acta (2006) 371:1–12. doi: 10.1016/j.cca.2006.02.016
CrossRef Full Text | Google Scholar
48. Koivisto O, Hanel A, Carlberg C. Cardinal vitamin D target genes with functions in the immune system. Nutrients (2020) 12:1140. doi: 10.3390/nu12041140
CrossRef Full Text | Google Scholar
49. Mazziotti G, Formenti AM, Frara South, Doga G, Giustina A. Vitamin D and Glucocorticoid-Induced Osteoporosis. Front Horm Res (2017) 50:149–lx. doi: 10.1159/000486078. Basel: Karger;.
CrossRef Total Text | Google Scholar
l. Gram J, Junker P, Nielsen HK, Bollerslev J. Furnishings of short-term handling with prednisolone and calcitriol on bone and mineral metabolism in normal men. Os (1998) 23:297–302. doi: 10.1016/S8756-3282(98)00097-0
CrossRef Full Text | Google Scholar
51. Dhawan P, Christakos S. Novel regulation of 25-hydroxyvitamin D3 24-hydroxylase (24(OH)ase) transcription past glucocorticoids: Cooperative furnishings of the glucocorticoid receptor, C/EBPβ, and the vitamin D receptor in 24(OH)ase transcription. J Jail cell Biochem (2010) 110:1314–23. doi: 10.1002/jcb.22645
CrossRef Full Text | Google Scholar
52. Hidalgo AA, Trump DL, Johnson CS. Glucocorticoid regulation of the vitamin D receptor. J Steroid Biochem Mol Biol (2010) 121:372–5. doi: x.1016/j.jsbmb.2010.03.081
CrossRef Full Text | Google Scholar
53. Chiodini I, Adda Thousand, Scillitani A, Coletti F, Morelli V, Di Lembo Due south, et al. Cortisol secretion in patients with blazon two diabetes: Relationship with chronic complications. Diabetes Intendance (2007) 30:83–8. doi: 10.2337/dc06-1267
CrossRef Full Text | Google Scholar
54. Yi B, Huang J, Zhang W, Li AM, Yang SK, Sunday J, et al. Vitamin D receptor down-regulation is associated with severity of albuminuria in type ii diabetes patients. J Clin Endocrinol Metab (2016) 101:4395–404. doi: 10.1210/jc.2016-1516
CrossRef Total Text | Google Scholar
55. Godschalk M, Levy JR, Downs RWJ. Glucocorticoids decrease vitamin D receptor number and gene expression in human osteosarcoma cells. J Bone Miner Res (1992) 7:21–7. doi: 10.1002/jbmr.5650070105
CrossRef Total Text | Google Scholar
56. Fleet JC, Schoch RD. Molecular mechanisms for regulation of intestinal calcium absorption by vitamin D and other factors. Crit Rev Clin Lab Sci (2010) 47:181–95. doi: 10.3109/10408363.2010.536429
CrossRef Full Text | Google Scholar
57. Gilad LA, Bresler T, Gnainsky J, Smirnoff P, Schwartz B. Regulation of vitamin D receptor expression via estrogen-induced activation of the ERK 1/ii signaling pathway in colon and breast cancer cells. J Endocrinol (2005) 185:577–92. doi: 10.1677/joe.1.05770
CrossRef Total Text | Google Scholar
58. Raval-Pandya K, Freedman LP, Li H, Christakos Southward. Thyroid Hormone Receptor Does Not Heterodimerize with the Vitamin D Receptor but Represses Vitamin D Receptor-Mediated Transactivation. Mol Endocrinol (1998) 12:1367–79. doi: ten.1210/mend.12.nine.0165
CrossRef Full Text | Google Scholar
59. Ciulei G, Orasan OH, Coste SC, Cozma A, Negrean Five, Procopciuc LM. Vitamin D and the insulin-similar growth factor organisation: Implications for colorectal neoplasia. Eur J Clin Invest (2020) fifty:e13265. doi: x.1111/eci.13265
CrossRef Full Text | Google Scholar
60. Deng C, Ueda Eastward, Chen KE, Bula C, Norman AW, Luben RA, et al. Prolactin blocks nuclear translocation of VDR by regulating its interaction with BRCA1 in osteosarcoma cells. Mol Endocrinol (2009) 23:226–36. doi: 10.1210/me.2008-0075
CrossRef Full Text | Google Scholar
61. Pramanik R, Asplin JR, Lindeman C, Favus MJ, Bai S, Coe FL. Lipopolysaccharide negatively modulates vitamin D activity by down-regulating expression of vitamin D-induced VDR in human monocytic THP-i cells. Cell Immunol (2004) 232:137–43. doi: 10.1016/j.cellimm.2005.03.004
CrossRef Full Text | Google Scholar
62. Malloy PJ, Feldman D. Inactivation of the human vitamin D receptor by caspase-3. Endocrinology (2009) 150:679–86. doi: 10.1210/en.2008-1217
CrossRef Full Text | Google Scholar
63. Wall DM, Mccormick BA. Bacterial secreted effectors and caspase-3 interactions. Cell Microbiol (2014) 16:1746–56. doi: 10.1111/cmi.12368
CrossRef Full Text | Google Scholar
64. Salazar JC, Duhnam-European monetary system S, La Vake C, Cruz AR, Moore MW, Caimano MJ, et al. Activation of human monocytes past live Borrelia burgdorferi generates TLR2-dependent and -independent responses which include consecration of IFN-β. PloS Pathog (2009) v:e1000444. doi: ten.1371/journal.ppat.1000444
CrossRef Full Text | Google Scholar
65. Morgan JW, Reddy GS, Uskokovic MR, May BK, Omdahl JL, Maizel AL, et al. Functional block for 1α,25-dihydroxyvitamin D3-mediated gene regulation in human B lymphocytes. J Biol Chem (1994) 269:13437–43. doi: x.1016/S0021-9258(17)36851-five
CrossRef Full Text | Google Scholar
66. Yenamandra SP, Lundin A, Arulampalam V, Yurchenko M, Pettersson S, Klein G, et al. Expression profile of nuclear receptors upon Epstein - Barr virus induced B cell transformation. Exp Oncol (2009) 31:92–half dozen.
Google Scholar
67. Yenamandra SP, Hellman U, Kempkes B, Darekar SD, Petermann S, Sculley T, et al. Epstein-Barr virus encoded EBNA-3 binds to vitamin D receptor and blocks activation of its target genes. Cell Mol Life Sci (2010) 67:4249–56. doi: ten.1007/s00018-010-0441-4
CrossRef Full Text | Google Scholar
68. Rieder FJJ, Gröschel C, Kastner MT, Kosulin K, Laengle J, Zadnikar R, et al. Human cytomegalovirus infection downregulates vitamin-D receptor in mammalian cells. J Steroid Biochem Mol Biol (2017) 165:356–62. doi: 10.1016/j.jsbmb.2016.08.002
CrossRef Full Text | Google Scholar
69. Getts DR, Chastain EML, Terry RL, Miller SD. Virus infection, antiviral immunity, and autoimmunity. Immunol Rev (2013) 255:197–209. doi: x.1111/imr.12091
CrossRef Full Text | Google Scholar
lxx. Tam CC, O'Brien SJ, Petersen I, Islam A, Hayward A, Rodriguez LC. Guillain-Barré syndrome and preceding infection with Campylobacter, influenza and Epstein-Barr virus in the Full general Practise Research Database. PloS One (2007) 2:e344. doi: x.1371/periodical.pone.0000344
CrossRef Full Text | Google Scholar
71. Giovannoni G, Cutter GR, Lunemann J, Martin R, Münz C, Sriram S, et al. Infectious causes of multiple sclerosis. Lancet Neurol (2006) 5:887–94. doi: ten.1016/S1474-4422(06)70577-4
CrossRef Full Text | Google Scholar
72. Barragry JM, France MW, Corless D, Gupta SP, Switala S, Boucher BJ, et al. Intestinal cholecalciferol absorption in the elderly and in younger adults. Clin Sci Mol Med (1978) 55:213–20. doi: 10.1042/cs0550213
CrossRef Full Text | Google Scholar
73. Maclaughlin J, Holick MF, Kasper K. Aging Decreases the Capacity of Human Skin to Produce Vitamin D3. Nutr Clin Pract (1986) 1:57–8. doi: 10.1177/088453368600100118
CrossRef Total Text | Google Scholar
75. Mold Thou, Chmielecka A, Rodriguez MRR, Thom F, Linhart C, King A, et al. Aluminium in brain tissue in multiple sclerosis. Int J Environ Res Public Wellness (2018) 15:1777. doi: 10.3390/ijerph15081777
CrossRef Full Text | Google Scholar
76. Henry HL, Norman AW. Interactions Betwixt Aluminum and the Actions and Metabolism of Vitamin D3 in the Chick. Calcif Tissue Int (1985) 37:484–90. doi: 10.1007/BF02557831
CrossRef Full Text | Google Scholar
77. Ifergan I, Kebir H, Terouz Southward, Alvarez JI, Lécuyer MA, Gendron S, et al. Role of Ninjurin-one in the migration of myeloid cells to central nervous system inflammatory lesions. Ann Neurol (2011) 70:751–63. doi: 10.1002/ana.22519
CrossRef Total Text | Google Scholar
78. Carlberg C, Seuter Due south, Nurmi T, Tuomainen TP, Virtanen JK, Neme A. In vivo response of the human epigenome to vitamin D: A Proof-of-principle written report. J Steroid Biochem Mol Biol (2018) 180:142–8. doi: x.1016/j.jsbmb.2018.01.002
CrossRef Full Text | Google Scholar
79. Lemke D. Die Bedeutung von Lebensstilfaktoren für die Sekundärprävention der Multiplen Sklerose. [Medical thesis]. Federal republic of germany: University of Mainz (2019).
Google Scholar
fourscore. Ramagopalan SV, Maugeri NJ, Handunnetthi L, Lincoln MR, Orton SM, Dyment DA, et al. Expression of the multiple sclerosis-associated MHC grade 2 allele HLA-DRB1*1501 is regulated by vitamin D. PloS Genet (2009) 5:e1000369. doi: ten.1371/journal.pgen.1000369
CrossRef Full Text | Google Scholar
81. González-Sancho JM, Larriba MJ, Muñoz A. Wnt and vitamin D at the crossroads in solid cancer. Cancers (Basel) (2020) 12:3434. doi: 10.3390/cancers12113434
CrossRef Total Text | Google Scholar
82. Fernández-Barral A, Bustamante-Madrid P, Ferrer-Mayorga M, Barbáchano A, Larriba MJ, Muñoz A. Vitamin D effects on cell differentiation and stemness in cancer. Cancers (Basel) (2020) 12:2413. doi: 10.3390/cancers12092413
CrossRef Total Text | Google Scholar
83. Vidigal VM, Silva TD, de Oliveira J, Pimenta CAM, Felipe AV, Forones NM. Genetic polymorphisms of vitamin D receptor (VDR), CYP27B1 and CYP24A1 genes and the take a chance of colorectal cancer. Int J Biol Markers (2017) 32:e224–30. doi: 10.5301/jbm.5000248
CrossRef Full Text | Google Scholar
84. Gnagnarella P, Raimondi Southward, Aristarco V, Johansson HA, Bellerba F, Corso F, et al. Vitamin D Receptor Polymorphisms and Cancer. Adv Exp Med Biol (2020) 1268:53–114. doi: 10.1007/978-3-030-46227-7_4
CrossRef Full Text | Google Scholar
85. Zur Hausen H. Condylomata acuminata and human genital cancer. Cancer Res (1976) 36:1–2.
Google Scholar
86. zur Hausen H. Infections Causing Human Cancer. 1st ed. Weinheim, Deutschland: Wiley-VCH Verlag GmbH & Co (2006).
Google Scholar
87. Kedia R, Desouza C, Smith LM, Shivaswamy V. A retrospective review of insulin requirements in patients using U-500 insulin hospitalized to a Veterans Affairs Infirmary. J Diabetes Complications (2017) 31:874–nine. doi: 10.1016/j.jdiacomp.2017.02.008
CrossRef Full Text | Google Scholar
88. Lutz N, Steffen P, Nigg L. Nebenwirkung einer nicht evidenzbasierten MS-Therapie. Swiss Med Forum - Schweizerisches Medizin-Forum (2020) twenty:230–3. doi: 10.4414/smf.2020.08365
CrossRef Full Text | Google Scholar
Source: https://www.frontiersin.org/articles/10.3389/fimmu.2021.655739/full
0 Response to "Can Vitamin D Cause Gas in a Baby"
إرسال تعليق